Abstract
Background:
The ATHNdataset is a HIPAA-compliant, de-identified database, sponsored by the American Thrombosis and Hemostasis Network. Over 40,000 people with a bleeding or clotting disorder followed at United States hemophilia treatment centers (HTC) have submitted health information, including 15,747 hemophilia A patients.
Aims:
To evaluate the effectiveness of damoctocog alfa pegol (BAY 94-9027/Jivi®) to treat hemophilia A adolescent patients aged between 12-18 years in a real-world setting.
Methods:
The ATHNdataset was queried for people with hemophilia A aged ≥12 years of age treated with damoctocog alfa pegol. From this dataset adolescent patients aged between 12-18 years, treated either prophylactically or on-demand were identified. Collected data included baseline demographic data, treatment history, comorbidities and bleeding rates. The data were captured via patient chart review and entered into the database. The database was queried for patient data between January 1, 2010 and October 31, 2020.
Results:
At the data cutoff, 16 patients aged between 12-18 years were being treated with damoctocog alfa pegol. In this group, 13 patients (81%) had severe hemophilia A and 3 patients (19%) had mild/moderate disease. The majority of patients were male - 14/16 (87.5%) with 2/16 (12.5%) being female and the mean age of patients was 15.9 years old (median = 15.8). Of the total, 4 patients (25%) had a history of inhibitors. Patients had an average of 1.2 years of therapy with damoctocog alfa pegol and most (56%) had over 12 months of treatment.
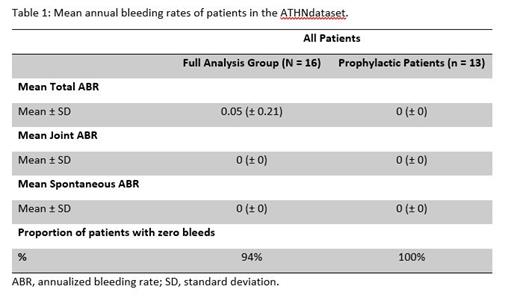
All the patients included in this analysis had documented bleeding rates in the ATHN system (Table 1). Mean total annualized bleed rate (ABR) was 0.05. Mean joint ABR and spontaneous ABR were zero. In the subgroup of patients who were on prophylaxis with damoctocog alfa pegol (n = 13), the mean total ABR, joint ABR and spontaneous ABR were zero. The proportion of patients with zero total bleeds during the entire observational period was 15/16 (94%) in the overall cohort and 13/13 (100%) in adolescent patients who were on prophylaxis. The majority of patients who were on prophylaxis 9/13 (69%) were treated twice-weekly with the mean weekly dose of 77.2 IU/kg/wk.
Conclusions:
The data from this analysis show that damoctocog alfa pegol provides protection from bleeding events in adolescent patients aged 12-18 years in the real-world setting. Mean ABR was low in this patient population, with 94% of patients experiencing zero bleeds. This data should be interpreted with caution due to limitations in real-world studies.
Recht: American Thrombosis and Hemostasis Network: Current Employment; Oregon Health & Science University: Current Employment; Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders: Speakers Bureau; uniQure: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; Pfizer: Consultancy; Octapharma: Consultancy; Novo Nordisk: Consultancy; Kedrion: Consultancy; Hema Biologics: Consultancy; Genentech: Consultancy; CSL Behring: Consultancy; Catalyst Biosciences: Consultancy. He: ATHN: Ended employment in the past 24 months. Moulton: Bayer: Current Employment. Charafi: Bayer: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal